Our Science
The Expansive Potential of Pelareorep
Cancer has evolved myriad mechanisms to evade immune detection, blunting the therapeutic effect of a broad range of drugs developed to treat it. Pelareorep is a first-in-class, nonpathogenic, oncolytic virus that can be delivered intravenously and works by generating, recruiting, and training immune cells to recognize and kill cancer while remodeling the tumor microenvironment to enable immune cell access. In addition to its demonstrated single-agent activity, pelareorep can also work in synergy with chemotherapy, immune checkpoint inhibitors (ICIs), CAR T-cell therapy, proteasome inhibitors, bispecific antibodies, and CDK4/6 and PARP inhibitors to enhance its antitumor potential and meaningfully extend patient remissions.
Pelareorep specifically targets cancer cells and induces a cascade of inflammatory responses that activate the innate and adaptive immune system to destroy the tumor while sparing normal tissue. Pelareorep’s unique ability to introduce double-stranded RNA, a powerful immune stimulant, directly into cancer cells results in PD-L1 upregulation and cytokine and chemokine production, inducing enhanced infiltration and T-cell activation. Because pelareorep replicates only in tumor cells, it is well-tolerated by patients.
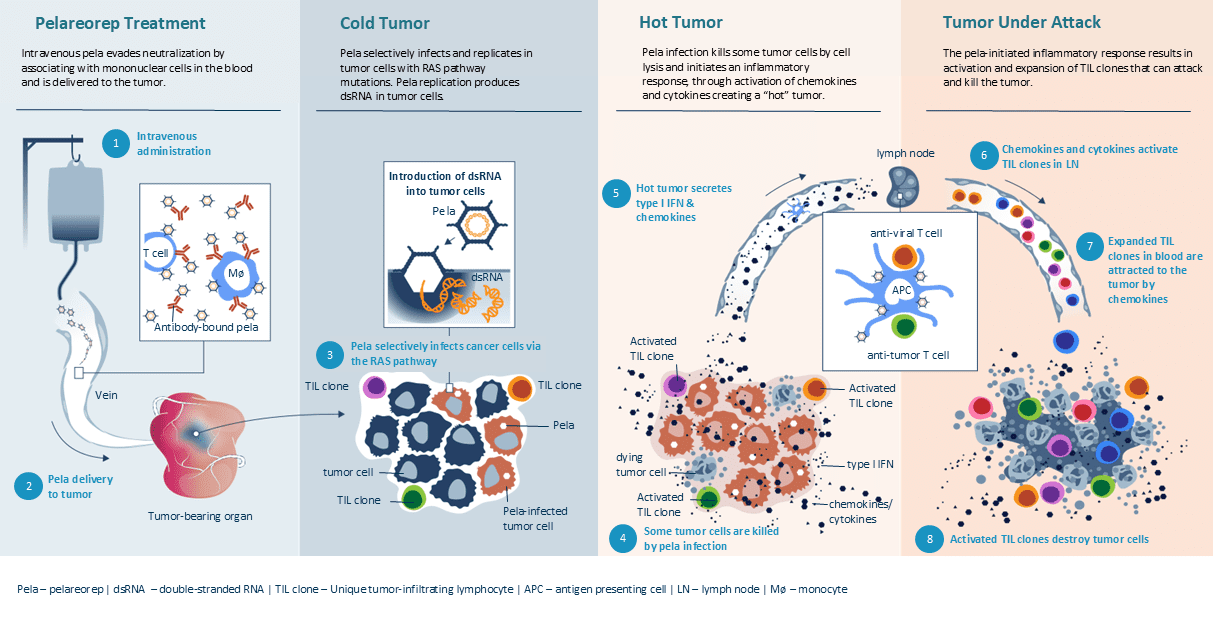
Intravenous pela evades neutralization by associating with mononuclear cells in the blood and is delivered to the tumor.
Cold Tumor
Pela selectively infects and replicates in tumor cells with RAS pathway mutations. Pela replication produces dsRNA in tumor cells.
Pela infection kills some tumor cells by cell lysis and initiates an inflammatory response, through activation of chemokines and cytokines creating a “hot” tumor.
Tumor Under Attack
The pela-initiated inflammatory response results in activation and expansion of TIL clones that can attack and kill the tumor.
A Case Study of Combination Therapy
Cancer cells evade immune destruction by signaling to the immune system that they do not need to be destroyed. Since immune evasion is a feature across oncologic disease, ICIs such as anti–PD-(L)1s can be applied to a broad range of cancers to facilitate tumor clearance. However, as few as 12.5% of patients see results with currently available ICIs.4
Pelareorep’s intravenous administration enables it to work systemically to unmask cancer cells, thereby allowing the immune system to identify and target them for destruction. On its own, pelareorep has the potential to treat primary and metastatic cancers. When added to existing treatment regimens, pelareorep can bolster their efficacy, offering patients the hope of longer, healthier lives.
Demonstrated Efficacy
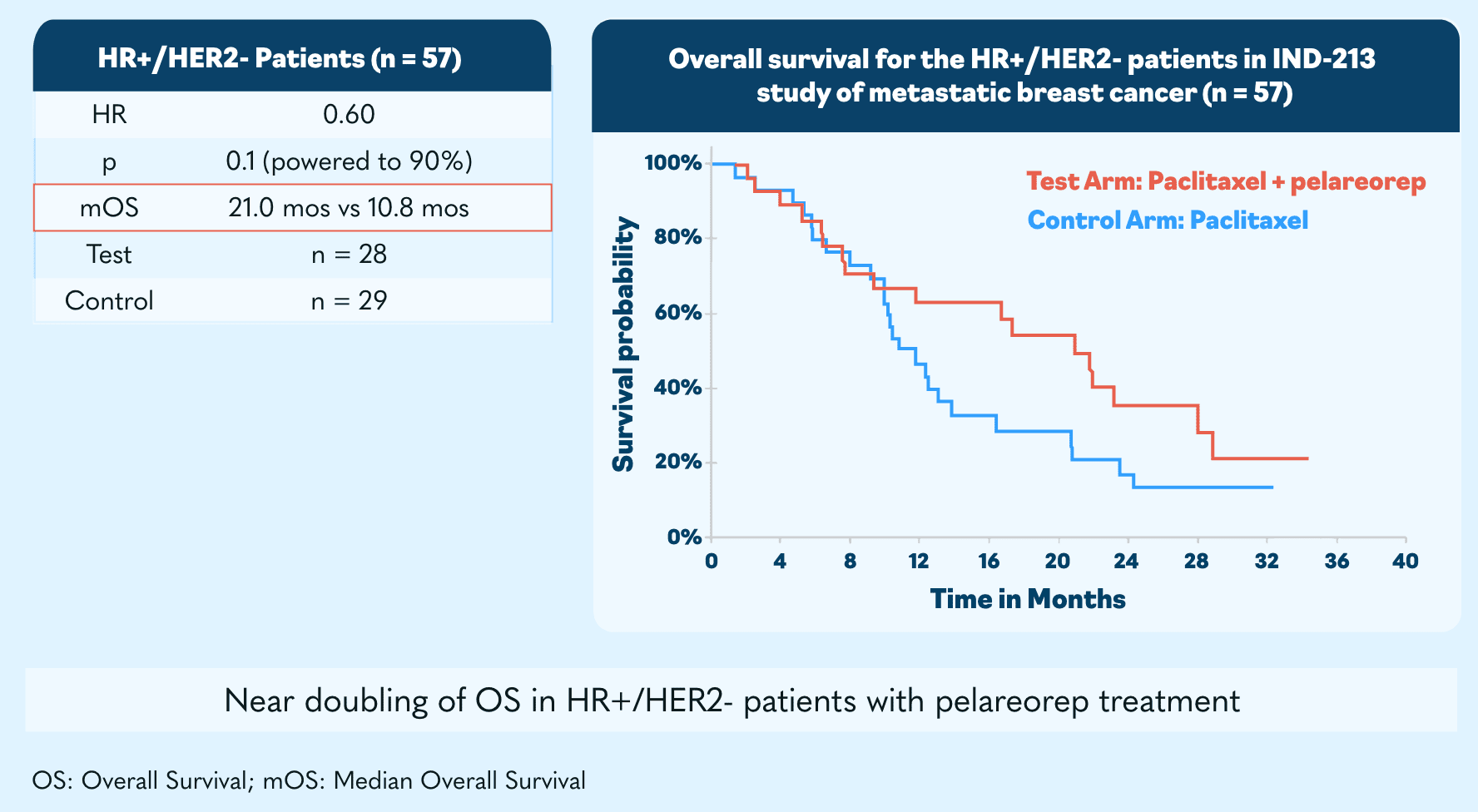
Metastatic breast cancer
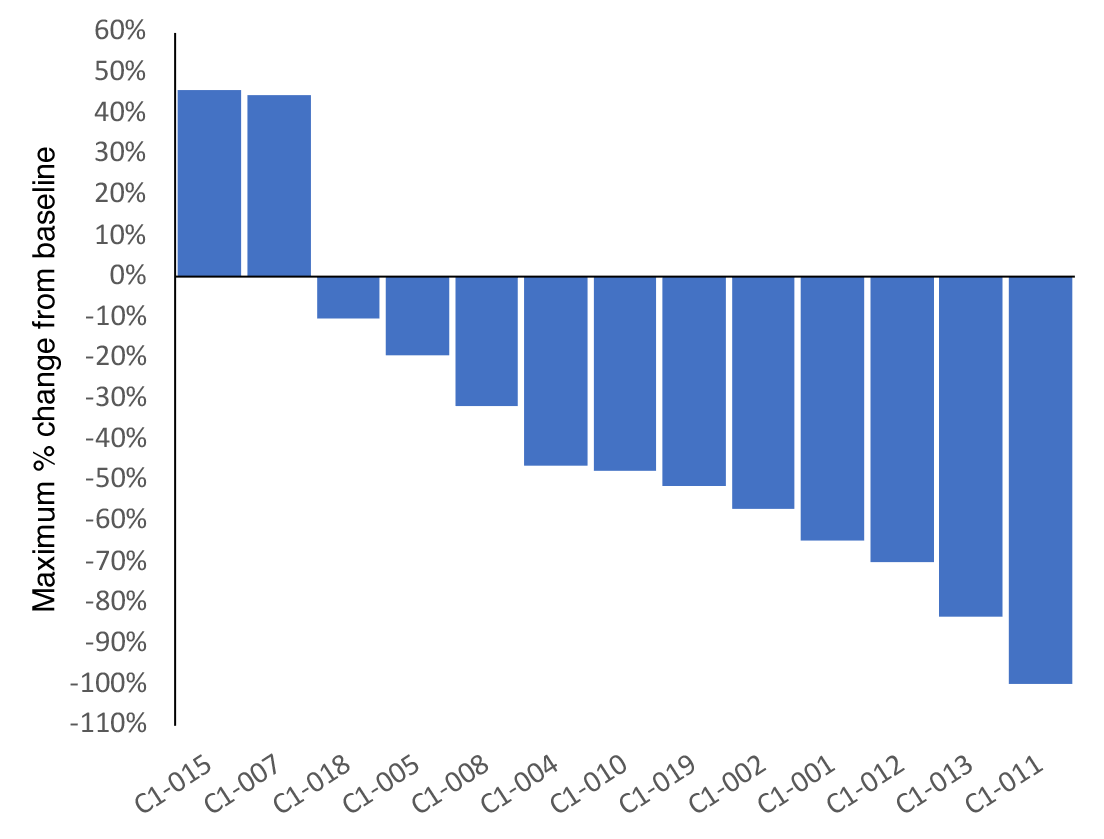
Pancreatic cancer
Pancreatic cancer is the fourth leading cause of cancer-related death in the United States, with fewer than 10% of those diagnosed surviving beyond 5 years.4 While advances in immunotherapy have revolutionized the treatment of many cancers, pancreatic cancer has continued to present a challenge, with pancreatic tumors being poorly immunogenic and thus showing little to no response to anti–PD‐(L)1 and anticytotoxic T‐lymphocyte–associated antigen 4 (anti–CTLA‐4) therapies.5
Updated clinical data showed a 62% objective response rate, which is more than double historical control trial averages.
- Haslam A, Gill J, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for Immune Checkpoint Inhibitor Drugs. JAMA Netw Open. 2020;3(3):e200423. Published 2020 Mar 2. doi:10.1001/jamanetworkopen.2020.0423
- SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. Surveillance Research Program, National Cancer Institute. Accessed September 27, 2022. https://seer.cancer.gov/explorer/
- Chun-Yu Liu, Chia-Yun Wu, Karineh Petrossian, Tzu-Ting Huang, Ling-Ming Tseng, Shiuan Chen, Treatment for the endocrine resistant breast cancer: Current options and future perspectives, The Journal of Steroid Biochemistry and Molecular Biology, Volume 172, 2017, Pages 166-175, ISSN 0960-0760, https://doi.org/10.1016/j.jsbmb.2017.07.001.
- Lee DH, Jang JY, Kang JS, et al. Recent treatment patterns and survival outcomes in pancreatic cancer according to clinical stage based on single-center large-cohort data. Ann Hepatobiliary Pancreat Surg. 2018;22(4):386-396. doi:10.14701/ahbps.2018.22.4.386
- Manji GA, Olive KP, Saenger YM, Oberstein P. Current and emerging therapies in metastatic pancreatic cancer. Clin Cancer Res. 2017;23(7):1670-1678. doi:10.1158/1078-0432.CCR-16-2319
Mechanism of action (MOA)
Pelareorep’s MOA has been validated in clinical studies by measuring T-cell infiltration, PD-L1 expression, and other effects of pelareorep treatment on tumors. The ability of pelareorep to turn “cold” tumors “hot” has been established in multiple tumor types including breast cancer and pancreatic cancer. By turning “cold” tumors “hot,” pelareorep makes tumors more easily recognized by the immune system, thus enhancing the effectiveness of oncology treatments like chemotherapy or ICIs.
Broad potential
Ongoing studies are evaluating pelareorep for a variety of indications, including HR+/HER2- breast cancer, advanced pancreatic cancer, metastatic colorectal cancer, unresectable anal cancer, triple-negative breast cancer, and multiple myeloma.
Contact Us
For questions regarding our science, partnership opportunities, or other inquiries, we encourage you to reach out.